Noble-gas notation: [ar]3s1 [he]2s22p63s1 [ne]3s23p4 [ne]3s1 – Noble-gas notation, an ingenious shorthand for representing electron configurations, offers a simplified yet powerful tool for understanding the electronic structures and chemical properties of elements. This notation, employing the electron configurations of noble gases as building blocks, provides a concise and systematic approach to deciphering the complexities of atomic structures.
By harnessing the stability and inertness of noble gases, noble-gas notation enables us to comprehend the electron configurations of elements with remarkable clarity. It serves as a gateway to unraveling the periodic trends, chemical reactivity, and diverse applications of elements in the realm of chemistry.
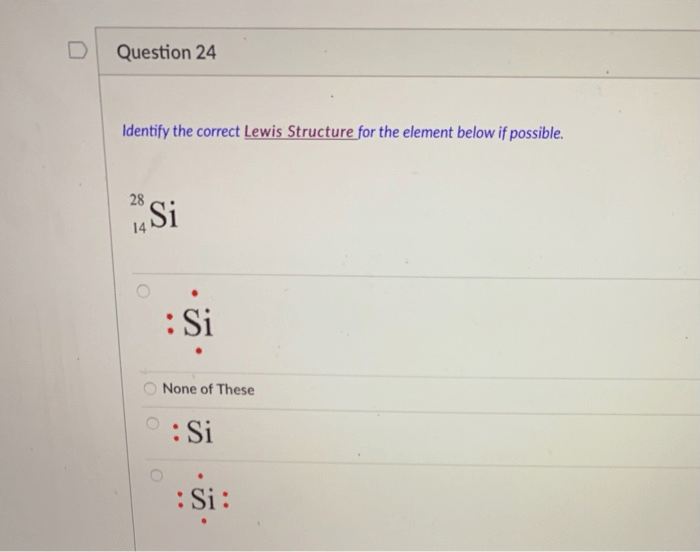
Noble-Gas Notation
![Gas core inert sodium notation noble Noble-gas notation: [ar]3s1 [he]2s22p63s1 [ne]3s23p4 [ne]3s1](https://ingrid.perka.org/wp-content/uploads/sites/425/2024/05/Noble-Gas-Configuration-1024x683-1.png)
Noble-gas notation is a method of representing the electron configuration of an element by using the electron configuration of a noble gas.
For example, the noble-gas notation for sodium is [Ne]3s 1. This means that sodium has the same electron configuration as neon, except that it has one additional electron in the 3s orbital.
Electron Configuration, Noble-gas notation: [ar]3s1 [he]2s22p63s1 [ne]3s23p4 [ne]3s1
Noble gases have a stable electron configuration, which is why they are unreactive.
The noble-gas notation simplifies the electron configuration of elements by using the symbol of the noble gas that has the same number of electrons as the element’s core electrons.
Periodic Table
Noble gases are located in Group 18 of the periodic table.
The noble-gas notation is useful for understanding the periodic table because it shows the relationship between the electron configuration of an element and its position in the periodic table.
Chemical Properties
Noble gases are chemically inert, which means that they do not react with other elements.
The noble-gas notation helps predict the chemical reactivity of elements because it shows the number of valence electrons an element has.
Applications
Noble gases are used in a variety of applications, such as:
- Lighting
- Welding
- Medical imaging
The noble-gas notation is useful for understanding these applications because it shows the properties of noble gases that make them useful for these applications.
Comparisons
Noble-gas notation is similar to other electron configuration notations, such as the orbital notation and the condensed electron configuration notation.
However, the noble-gas notation is more concise and easier to use than these other notations.
FAQ Overview: Noble-gas Notation: [ar]3s1 [he]2s22p63s1 [ne]3s23p4 [ne]3s1
What is noble-gas notation?
Noble-gas notation is a method of representing the electron configuration of an element by using the electron configuration of a noble gas followed by the remaining electrons in the element’s outermost energy level.
How does noble-gas notation simplify electron configuration?
Noble-gas notation simplifies electron configuration by using the electron configuration of a noble gas as a shorthand for the core electrons of the element. This allows us to focus on the outermost electrons, which are the most important for determining the element’s chemical properties.
How is noble-gas notation related to the periodic table?
Noble-gas notation is related to the periodic table because the noble gases are located in Group 18 of the periodic table. The number of valence electrons in an element is equal to the group number of the noble gas used in its noble-gas notation.
![Electron configurations configuration ppt powerpoint presentation Noble-gas notation: [ar]3s1 [he]2s22p63s1 [ne]3s23p4 [ne]3s1](https://ingrid.perka.org/wp-content/uploads/sites/425/2024/05/f42c4b45325b55c47cef234fa8e91ad0.jpg)